The Maillard reaction is a fundamental chemical reaction in coffee roasting, responsible for the formation of melanoidin—a dark brown compound—as well as a range of aromatic compounds. While often confused with sugar caramelization, the Maillard reaction involves an interaction between reducing sugars and amino compounds, such as amino acids or proteins, under heat. This results in non-enzymatic browning and the creation of complex flavors.
In English, the Maillard reaction is sometimes referred to as “sugar browning.” Within the roasting process, the time interval from when green beans begin turning yellow (referred to as the “color change”) to the first crack is commonly labeled the “Maillard Phase.”
Scientific Background
The reaction was first described by French chemist Louis-Camille Maillard. It proceeds in three primary stages: early, middle, and final, with reaction activity particularly strong in the range of 140°C to 165°C. In the early and middle phases, the Maillard reaction involves a series of complex transformations such as Amadori rearrangements and dehydration reactions. These transformations parallel the drying phase in coffee roasting.
In the final phase, high-molecular-weight, nitrogen-containing pigments called melanoidins are formed. These compounds exhibit strong antioxidant properties, and their concentration is generally proportional to roast color, as measured by absorbance or indicators such as L-value or Agtron score.
Aroma Generation by Amino Acids
During the Maillard reaction, a variety of volatile aroma compounds are generated, with the profile dependent on the type of amino acid and sugar involved. Below is a summary of the characteristic aromas produced by different amino acids at specific temperatures:
Valine
Chemical Formula: C5H11NO2
100°C: Rye bread aroma
180°C: Pungent chocolate aroma
Leucine
Chemical Formula: C6H13NO2
100°C: Sweet chocolate aroma
180°C: Aroma resembling cooked cheese
Isoleucine
Chemical Formula: C6H13NO2
100°C: Musty or mold-like aroma
180°C: Aroma resembling aged or cooked cheese
Phenylalanine
Chemical Formula: C9H11NO2
100°C: Violet aroma
180°C: Lilac aroma
Methionine
Chemical Formula: C5H11NO2S
100°C: Potato-like aroma
180°C: Potato-like aroma
Threonine
Chemical Formula: C4H9NO3
100°C: Chocolate aroma
180°C: Burnt or charred aroma
Histidine
Chemical Formula: C6H9N3O2
100°C: No significant aroma
180°C: Cornbread-like aroma
Aspartic Acid
Chemical Formula: C4H7NO4
100°C: Sugar candy aroma
180°C: Caramel-like aroma
Glutamine
Chemical Formula: C5H10N2O3
100°C: Chocolate aroma
180°C: Butterball aroma
Arginine
Chemical Formula: C6H14N4O2
100°C: Popcorn aroma
180°C: Burnt sugar aroma
Lysine
Chemical Formula: C6H14N2O2
100°C: No significant aroma
180°C: Bread-like aroma
Proline
Chemical Formula: C5H9NO2
100°C: Burnt protein aroma
180°C: Bread-like aroma
The summary of aroma compounds generated from various amino acids at specific temperatures, which serve as structural precursors of melanoidin, is based on findings reported in Issue 22 of the Journal of the Japan Institute for Food Behavior Science (CHA22).
CHA22 PDF (Japanese)
Strecker Degradation
The Maillard reaction is also accompanied by a secondary reaction known as Strecker degradation. This process was first identified by the German chemist Adolf Strecker and involves a reaction between α-dicarbonyl compounds and α-amino acids, resulting in the formation of aldehydes and pyrazines. As a consequence of this reaction, various aromatic compounds are generated, as illustrated in the following figure:
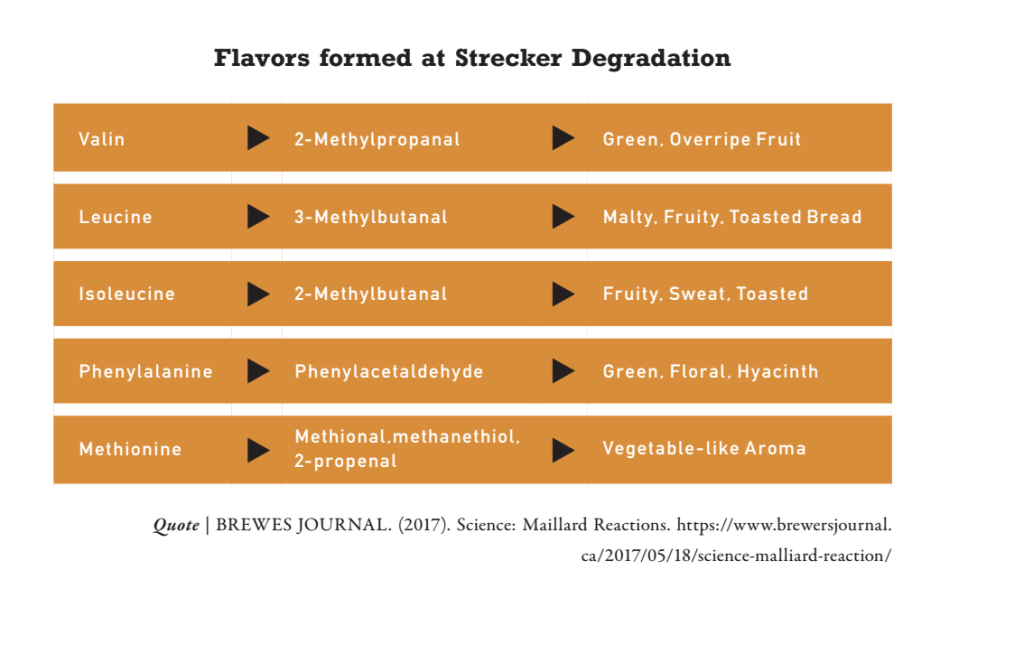
Adapted from The Coffee Fanatic, p.89 by Ryo Mikami (2025)
Conclusion
All amino acids share a structural basis involving hydrocarbon chains bonded to nitrogen and oxygen atoms, with variations in arrangement. Beyond melanoidin formation, amino acids themselves contribute to flavor through inherent bitterness, sweetness, or umami. Thus, a diversity of amino acids may contribute to the overall complexity of coffee flavor.
However, many of the aroma compounds formed through the Maillard reaction tend to fall within a limited sensory range—reminiscent of toasted, caramelized, or chocolate-like notes. Additional flavor development depends heavily on other compounds found in green coffee, such as organic acids (citric, malic, tartaric) and other precursors transformed during roasting. Importantly, not all roasting strategies that highlight unique acidity or floral tones require aggressive promotion of the Maillard reaction.
In summary, while the Maillard reaction plays a foundational role in coffee flavor development, it is only one part of the broader chemical transformation that occurs during roasting.

